
Exhibit 99.3

December 2019 | December 2019 Targeting Major Advances in Treatment of CNS Disorders Nasdaq: RLMD

December 2019 | 2 Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc . (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - looking statements . ” These statements can be identified by the fact that they do not relate strictly to historic or current facts . They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition . These statements are based upon the current beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties . Statements regarding future action, future performance and/or future results including, without limitation, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the forward - looking statements . Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate . Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement . It is not possible to predict or identify all such risks, contingencies and uncertainties . The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and financial performance . The Company assumes no obligation to update forward - looking statements as circumstances change . Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10-K, 10-Q and 8-K reports. Disclosures

December 2019 | Management team and scientific advisors have considerable CNS expertise Johnson & Johnson, Eli Lilly, Pfizer, Shire, Harvard, Yale, Cornell Multiple key catalysts expected over next 12 - 18 months* Presentation of REL - 1017 Phase 2 TRD study full data H1 2020 End of TRD Phase 2 meeting with the FDA H1 2020 Start of pivotal program in TRD H2 2020 Start of Phase 2 study in MDD H2 2020 Highly - compelling lead product opportunity w/ REL - 1017 Phase 2a TRD trial completed with positive results Statistically significant and rapid anti - depressant effects observed with favorable safety and tolerably profile Fast track designation from FDA Strong IP position around REL - 1017 with protection to the mid - 2030s REL - 1017 has potential in multiple underserved markets 1 Significant potential in multiple additional indications including Major Depressive Disorders (MDD) ~ 17.3M Americans suffered from MDD in 2017 1 ~ 10 to 30% of MDD patients suffer from Treatment Resistant Depression 2 3 Investment Highlights * Our fiscal year end is June 30. However the periods referred to in this slide are calendar years and quarters 1. https:// www.nimh.nih.gov /health/statistics/major - depression.shtml 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/

December 2019 | as a Potential Treatment for Depression d - Methadone (REL - 1017)

December 2019 | Compelling Lead Product Candidate: REL - 1017 • REL - 1017 is a non - competitive N - methyl - D - aspartate Receptor (NMDAR) antagonist • REL - 1017 has the potential to be the first oral NMDAR antagonist for the treatment of depression and treatment resistant depression • Completed Phase 1 and Phase 2 trial for treatment of Treatment Resistant Depression (TRD) • In a Phase 2a trial, both doses of REL - 1017 25 mg and 50 mg demonstrated rapid onset and sustained antidepressant effects with statistically significant differences compared to placebo on all efficacy measures • Study demonstrated rapid onset and long - lasting antidepressant effects • Only mild and moderate AEs - no serious AEs • No evidence of treatment induced dissociative an d psychotomimetic AEs • No evidence of opiate withdrawal symptoms in treatment groups vs placebo 5 REL - 1017 Program Overview Relmada is focused on advancing d - methadone (REL - 1017) as a rapid - acting oral treatment for depression and other CNS disorders.
December 2019 | Rapid onset 6 d - Methadone is an NMDAR antagonist with Significant Potential Advantages in the Treatment of Depression 1 Source: Am J Psychiatry. 2006 Nov;163(11):1905 - 17, https://www.samhsa.gov/data/sites/default/files/cbhsq - reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab8 - 10A Novel mechanism of action d - Methadone and other NMDA antagonists represent a new approach to treating depression with MOA markedly different from currently approved drugs (SSRIs, SNRIs, TCAs, MAOIs, etc . ) Faster onset of antidepressant activity – statistically significant difference in MADRS score vs placebo after 4 days of treatment Long lasting effect – statistically significant difference in MADRS score vs placebo seven days after termination of a 7 - day treatment Most of the currently approved products take up to a month to show antidepressant activity ~ 65 % MDD patients do not respond to first antidepressant treatment ~ 30 % MDD patients do not respond to up to 4 different antidepressant treatments Potentially equal or superior antidepressant effects with better safety profile than ketamine d - Methadone has the potential to address a high unmet need in MDD 1
December 2019 | 7 An Effective Treatment for Treatment - Resistant Depression (TRD) Remains a High Unmet Need ~10 to 30% of MDD patients suffers from TRD 1 ~17.3M U.S. MDD patients 2 1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/ 2. https://www.nimh.nih.gov/health/statistics/major - depression.shtml

December 2019 | 8 Expanding Focus on NMDAR’s Role in Treatment of Depression Johnson & Johnson Is Reinventing the Party Drug Ketamine to Treat Depression
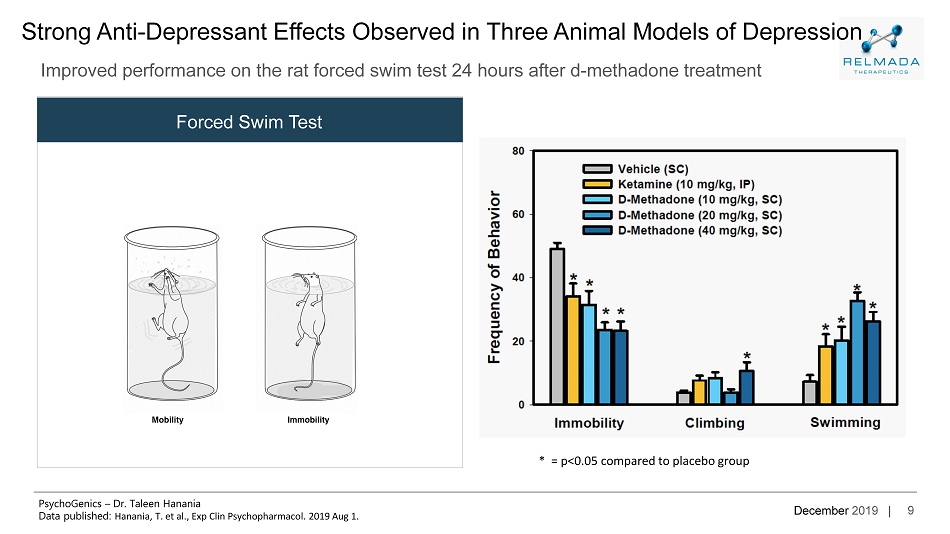
December 2019 | 9 Strong Anti - Depressant Effects Observed in Three Animal Models of Depression Improved performance on the rat forced swim test 24 hours after d - methadone treatment Forced Swim Test * = p<0.05 compared to placebo group PsychoGenics – Dr. Taleen Hanania Data published: Hanania , T. et al., Exp Clin Psychopharmacol . 2019 Aug 1.
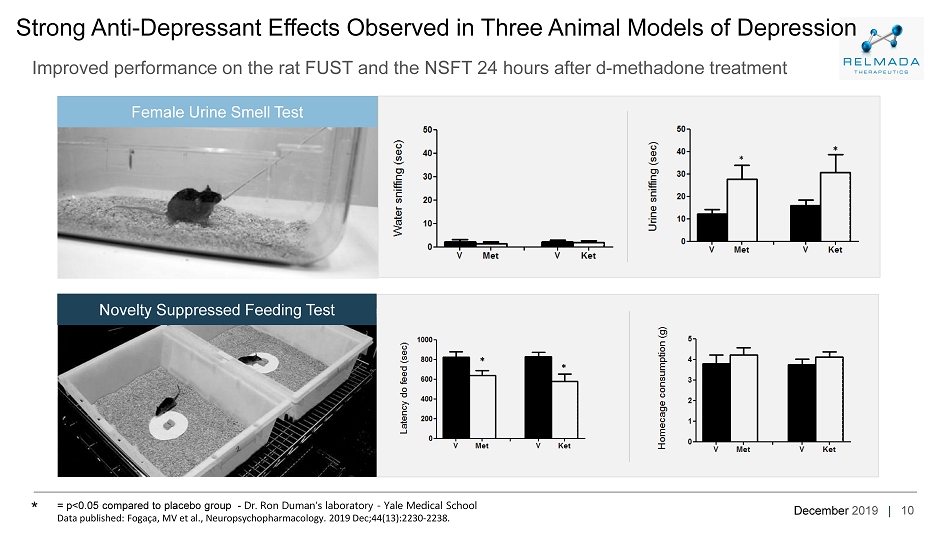
December 2019 | * * 10 Strong Anti - Depressant Effects Observed in Three Animal Models of Depression Improved performance on the rat FUST and the NSFT 24 hours after d - methadone treatment * * = p<0.05 compared to placebo group - Dr. Ron Duman’s laboratory - Yale Medical School Data published: Fogaça , MV et al., Neuropsychopharmacology. 2019 Dec;44(13):2230 - 2238. * Female Urine Smell Test Novelty Suppressed Feeding Test
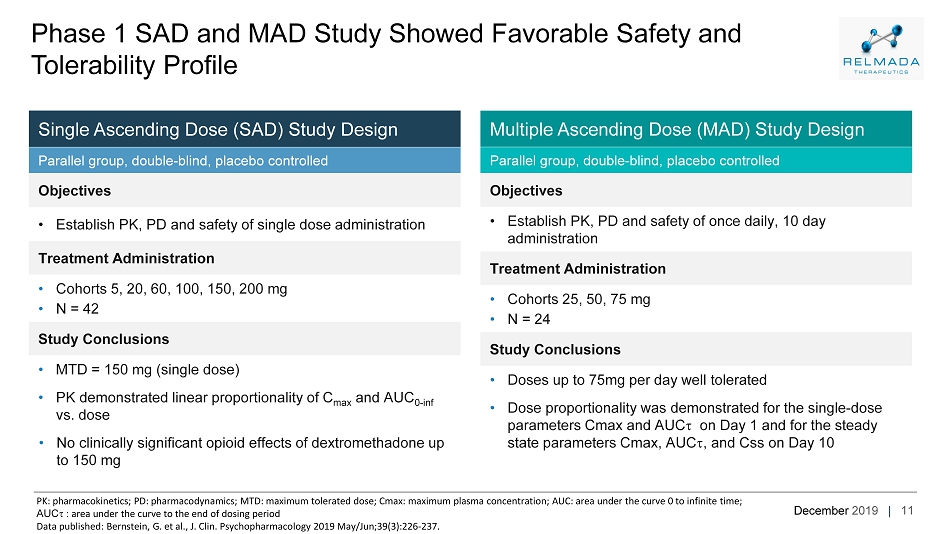
December 2019 | Multiple Ascending Dose (MAD) Study Design Parallel group, double - blind, placebo controlled Objectives • Establish PK, PD and safety of once daily, 10 day administration Treatment Administration • Cohorts 25, 50, 75 mg • N = 24 Study Conclusions • Doses up to 75mg per day well tolerated • Dose proportionality was demonstrated for the single - dose parameters Cmax and AUC t on Day 1 and for the steady state parameters Cmax, AUC t , and Css on Day 10 Single Ascending Dose (SAD) Study Design Parallel group, double - blind, placebo controlled Objectives • Establish PK, PD and safety of single dose administration Treatment Administration • Cohorts 5, 20, 60, 100, 150, 200 mg • N = 42 Study Conclusions • MTD = 150 mg (single dose) • PK demonstrated linear proportionality of C max and AUC 0 - inf vs. dose • No clinically significant opioid effects of dextromethadone up to 150 mg 11 Phase 1 SAD and MAD Study Showed Favorable Safety and Tolerability Profile PK: pharmacokinetics; PD: pharmacodynamics; MTD: maximum tolerated dose; Cmax : maximum plasma concentration; AUC: area under the curve 0 to infinite time; AUC t : area under the curve to the end of dosing period Data published: Bernstein, G. et al., J. Clin. Psychopharmacology 2019 May/Jun;39(3):226 - 237.
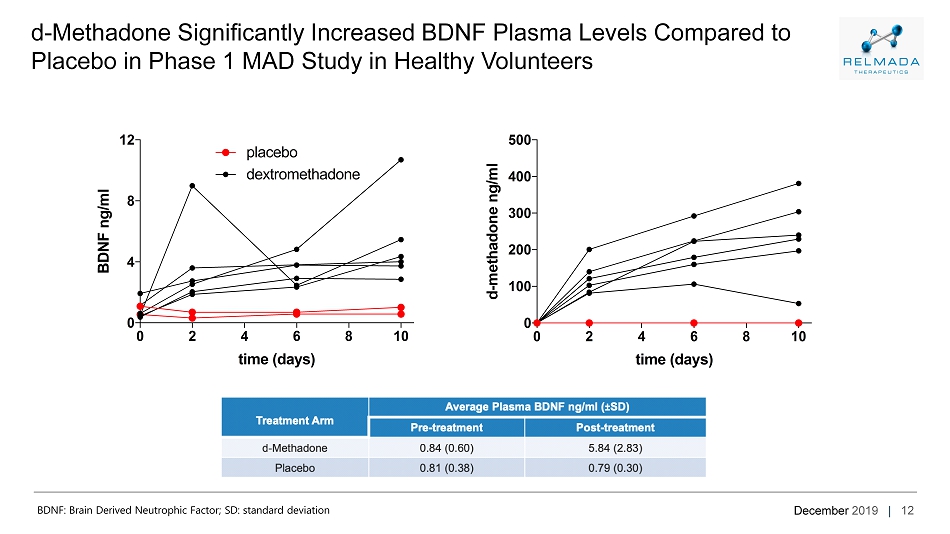
December 2019 | 12 d - Methadone Significantly Increased BDNF Plasma Levels Compared to Placebo in Phase 1 MAD Study in Healthy Volunteers 0 2 4 6 8 10 0 4 8 12 time (days) B D N F n g / m l placebo dextromethadone 0 2 4 6 8 10 0 100 200 300 400 500 time (days) d - m e t h a d o n e n g / m l 0 2 4 6 8 10 0 4 8 12 time (days) B D N F n g / m l placebo dextromethadone 0 2 4 6 8 10 0 100 200 300 400 500 time (days) d - m e t h a d o n e n g / m l BDNF: Brain Derived Neutrophic Factor; SD: standard deviation
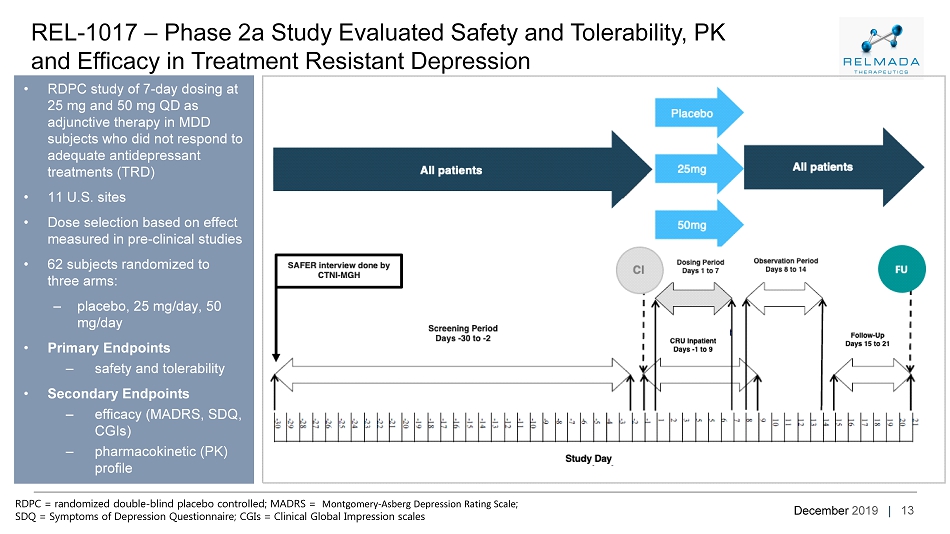
December 2019 | 13 REL - 1017 – Phase 2a Study Evaluated Safety and Tolerability, PK and Efficacy in Treatment Resistant Depression • RDPC study of 7 - day dosing at 25 mg and 50 mg QD as adjunctive therapy in MDD subjects who did not respond to adequate antidepressant treatments (TRD) • 11 U.S. sites • Dose selection based on effect measured in pre - clinical studies • 62 subjects randomized to three arms: – placebo, 25 mg/day, 50 mg/day • Primary Endpoints – safety and tolerability • Secondary Endpoints – efficacy (MADRS, SDQ, CGIs) – pharmacokinetic (PK) profile RDPC = randomized double - blind placebo controlled; MADRS = Montgomery - Asberg Depression Rating Scale ; SDQ = Symptoms of Depression Questionnaire; CGIs = Clinical Global Impression scales
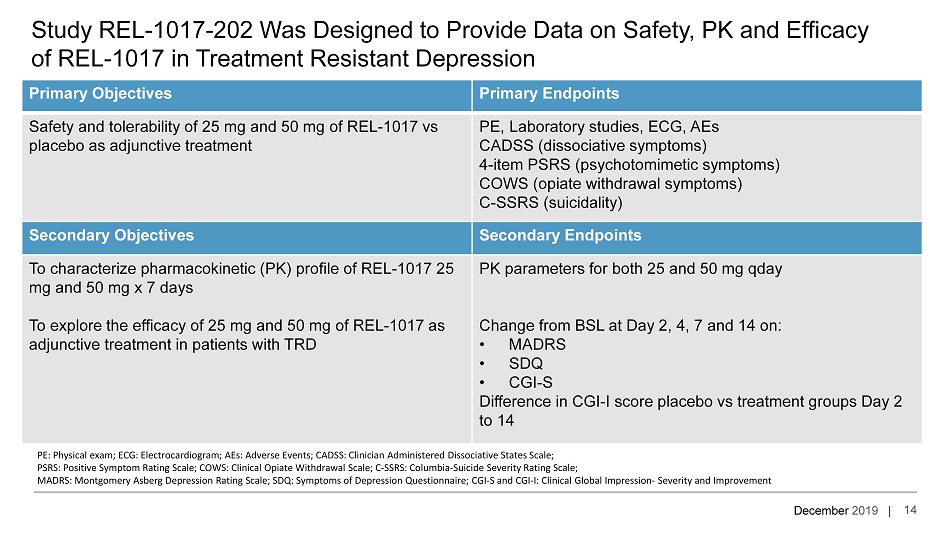
December 2019 | Study REL - 1017 - 202 Was Designed to Provide Data on Safety, PK and Efficacy of REL - 1017 in Treatment Resistant Depression Primary Objectives Primary Endpoints Safety and tolerability of 25 mg and 50 mg of REL - 1017 vs placebo as adjunctive treatment PE, Laboratory studies, ECG, AEs CADSS (dissociative symptoms) 4 - item PSRS (psychotomimetic symptoms) COWS (opiate withdrawal symptoms) C - SSRS (suicidality) Secondary Objectives Secondary Endpoints To characterize pharmacokinetic (PK) profile of REL - 1017 25 mg and 50 mg x 7 days To explore the efficacy of 25 mg and 50 mg of REL - 1017 as adjunctive treatment in patients with TRD PK parameters for both 25 and 50 mg qday Change from BSL at Day 2, 4, 7 and 14 on: • MADRS • SDQ • CGI - S Difference in CGI - I score placebo vs treatment groups Day 2 to 14 14 PE: Physical exam; ECG: Electrocardiogram; AEs: Adverse Events; CADSS: Clinician Administered Dissociative States Scale; PSRS: Positive Symptom Rating Scale; COWS: Clinical Opiate Withdrawal Scale; C - SSRS: Columbia - Suicide Severity Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; SDQ: Symptoms of Depression Questionnaire; CGI - S and CGI - I: Clinical Global Impression - Severity and I mprovement
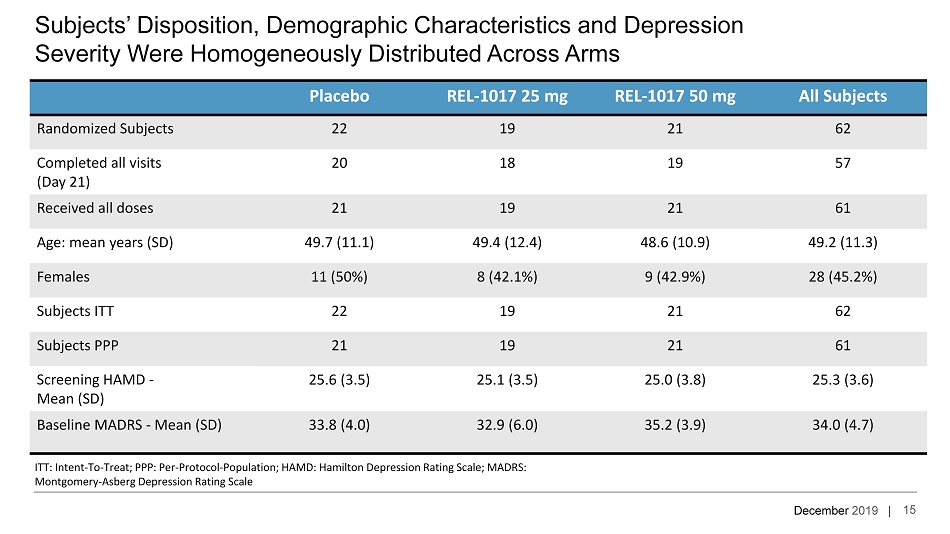
December 2019 | Placebo REL - 1017 25 mg REL - 1017 50 mg All Subjects Randomized Subjects 22 19 21 62 Completed all visits (Day 21) 20 18 19 57 Received all doses 21 19 21 61 Age: mean years (SD) 49.7 (11.1) 49.4 (12.4) 48.6 (10.9) 49.2 (11.3) Females 11 (50%) 8 (42.1%) 9 (42.9%) 28 (45.2%) Subjects ITT 22 19 21 62 Subjects PPP 21 19 21 61 Screening HAMD - Mean (SD) 25.6 (3.5) 25.1 (3.5) 25.0 (3.8) 25.3 (3.6) Baseline MADRS - Mean (SD) 33.8 (4.0) 32.9 (6.0) 35.2 (3.9) 34.0 (4.7) Subjects’ Disposition, Demographic Characteristics and Depression Severity Were Homogeneously Distributed Across Arms ITT: Intent - To - Treat; PPP: Per - Protocol - Population; HAMD: Hamilton Depression Rating Scale; MADRS: Montgomery - Asberg Depression Rating Scale 15
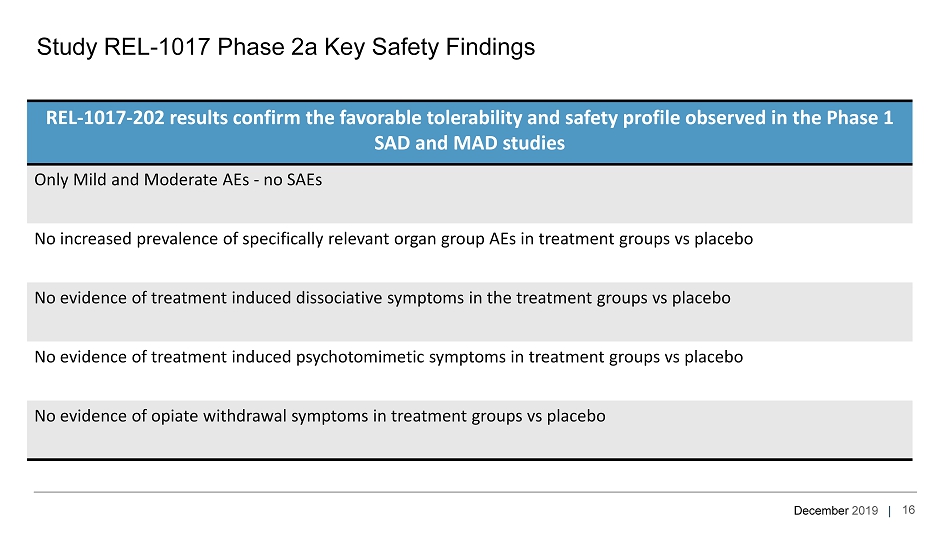
December 2019 | REL - 1017 - 202 results confirm the favorable tolerability and safety profile observed in the Phase 1 SAD and MAD studies Only Mild and Moderate AEs - no SAEs No increased prevalence of specifically relevant organ group AEs in treatment groups vs placebo No evidence of treatment induced dissociative symptoms in the treatment groups vs placebo No evidence of treatment induced psychotomimetic symptoms in treatment groups vs placebo No evidence of opiate withdrawal symptoms in treatment groups vs placebo Study REL - 1017 Phase 2a Key Safety Findings 16
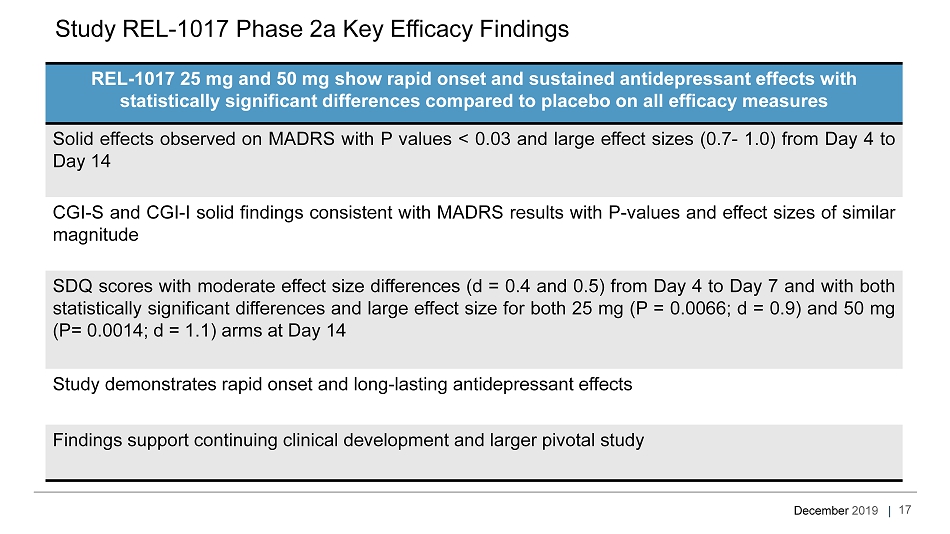
December 2019 | REL - 1017 25 mg and 50 mg show rapid onset and sustained antidepressant effects with statistically significant differences compared to placebo on all efficacy measures Solid effects observed on MADRS with P values < 0 . 03 and large effect sizes ( 0 . 7 - 1 . 0 ) from Day 4 to Day 14 CGI - S and CGI - I solid findings consistent with MADRS results with P - values and effect sizes of similar magnitude SDQ scores with moderate effect size differences (d = 0 . 4 and 0 . 5 ) from Day 4 to Day 7 and with both statistically significant differences and large effect size for both 25 mg (P = 0 . 0066 ; d = 0 . 9 ) and 50 mg (P= 0 . 0014 ; d = 1 . 1 ) arms at Day 14 Study demonstrates rapid onset and long - lasting antidepressant effects Findings support continuing clinical development and larger pivotal study Study REL - 1017 Phase 2a Key Efficacy Findings 17
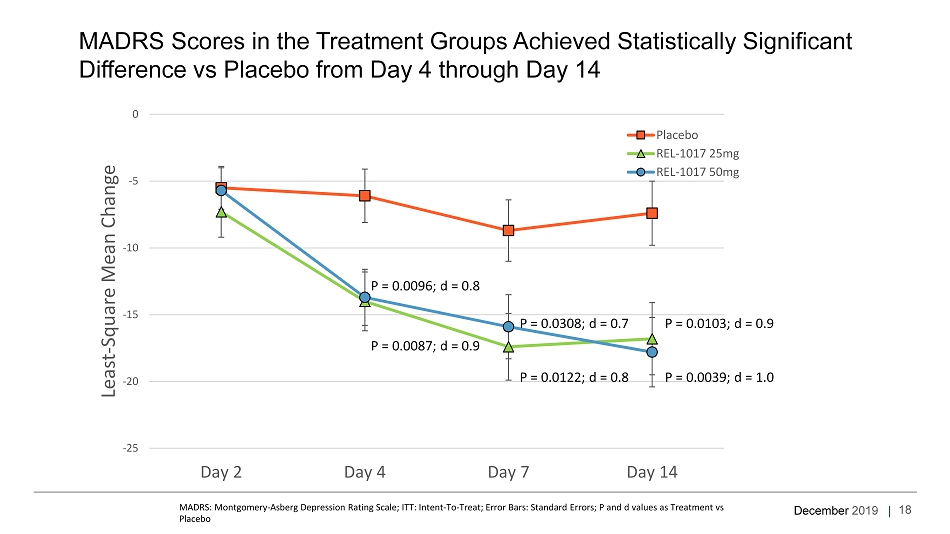
December 2019 | MADRS Scores in the Treatment Groups Achieved Statistically Significant Difference vs Placebo from Day 4 through Day 14 MADRS: Montgomery - Asberg Depression Rating Scale; ITT: Intent - To - Treat; Error Bars: Standard Errors; P and d values as Treatment vs Placebo -25 -20 -15 -10 -5 0 Day 2 Day 4 Day 7 Day 14 Least - Square Mean Change Placebo REL-1017 25mg REL-1017 50mg P = 0.0087; d = 0.9 P = 0.0096; d = 0.8 P = 0.0122; d = 0.8 P = 0.0308; d = 0.7 P = 0.0103; d = 0.9 P = 0.0039; d = 1.0 18
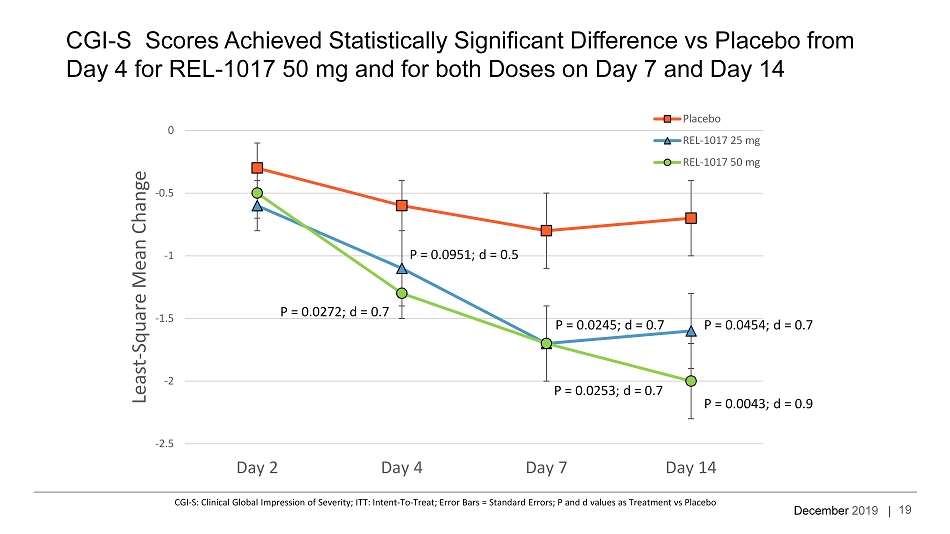
December 2019 | CGI - S Scores Achieved Statistically Significant Difference vs Placebo from Day 4 for REL - 1017 50 mg and for both Doses on Day 7 and Day 14 CGI - S: Clinical Global Impression of Severity; ITT: Intent - To - Treat; Error Bars = Standard Errors; P and d values as Treatment v s Placebo -2.5 -2 -1.5 -1 -0.5 0 Day 2 Day 4 Day 7 Day 14 Least - Square Mean Change Placebo REL-1017 25 mg REL-1017 50 mg P = 0.0272; d = 0.7 P = 0.0951; d = 0.5 P = 0.0253; d = 0.7 P = 0.0245; d = 0.7 P = 0.0454; d = 0.7 P = 0.0043; d = 0.9 19
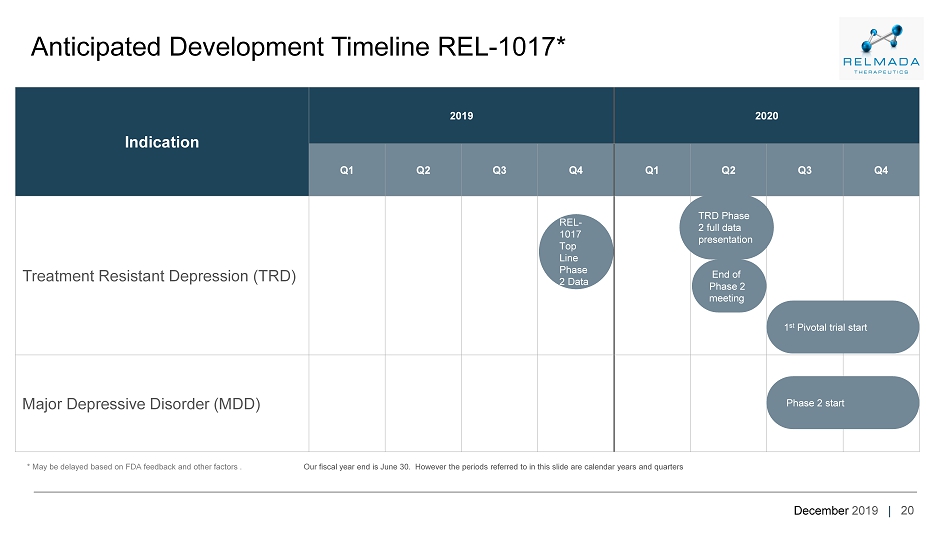
December 2019 | 20 Anticipated Development Timeline REL - 1017* * May be delayed based on FDA feedback and other factors . Indication 2019 2020 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Treatment Resistant Depression (TRD) Major Depressive Disorder (MDD) REL - 1017 Top Line Phase 2 Data End of Phase 2 meeting 1 st Pivotal trial start Phase 2 start Our fiscal year end is June 30. However the periods referred to in this slide are calendar years and quarters TRD Phase 2 full data presentation
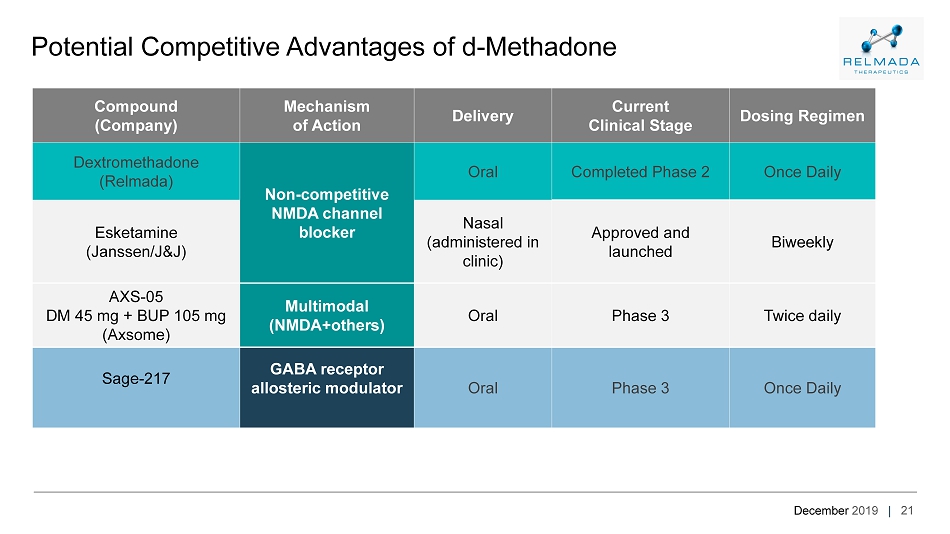
December 2019 | 21 Potential Competitive Advantages of d - Methadone Compound (Company) Mechanism of Action Delivery Current Clinical Stage Dosing Regimen Dextromethadone (Relmada) Non - competitive NMDA channel blocker Oral Completed Phase 2 Once Daily Esketamine (Janssen/J&J) Nasal (administered in clinic) Approved and launched Biweekly AXS - 05 DM 45 mg + BUP 105 mg ( Axsome ) Multimodal ( NMDA+others ) Oral Phase 3 Twice daily Sage - 217 GABA receptor allosteric modulator Oral Phase 3 Once Daily

December 2019 | Corporate Information

December 2019 | 23 Financial Overview Cash & Cash Equivalents (as of 9/30/19) $7.8 million Common Shares Outstanding (as of 11/8/19) ~10.3 million

December 2019 | Management team and scientific advisors have considerable CNS expertise Johnson & Johnson, Eli Lilly, Pfizer, Shire, Harvard, Yale, Cornell Multiple key catalysts expected over next 12 - 18 months* Presentation of REL - 1017 Phase 2 TRD study full data H1 2020 End of TRD Phase 2 meeting with the FDA H1 2020 Start of pivotal program in TRD H2 2020 Start of Phase 2 study in MDD H2 2020 Highly - compelling lead product opportunity w/ REL - 1017 Phase 2a TRD trial completed with positive results Statistically significant and rapid anti - depressant effects observed with favorable safety and tolerably profile Fast track designation from FDA Strong IP position around REL - 1017 with protection to the mid - 2030s REL - 1017 has potential in multiple underserved markets 1 Significant potential in multiple additional indications including Major Depressive Disorders (MDD) ~ 17.3M Americans suffered from MDD in 2017 1 ~ 10 to 30% of MDD patients suffer from Treatment Resistant Depression 2 24 Investment Highlights * Our fiscal year end is June 30. However the periods referred to in this slide are calendar years and quarters 1. https:// www.nimh.nih.gov /health/statistics/major - depression.shtml 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/

December 2019 | 25 Management Team and Key Scientific Advisors Management Sergio Traversa PharmD, MBA, Chief Executive Officer Ottavio Vitolo Senior Vice President, Head of R&D and Chief Medical Officer Chuck Ence Chief Financial Officer Advisors Maurizio Fava Scientific Advisor Charles Inturrisi Scientific Advisor Paolo Manfredi Scientific Advisor

December 2019 | December 2019 Targeting Major Advances in Treatment of CNS Disorders Nasdaq: RLMD